The microbiome is the collective genome of the microorganisms within a particular environment and the microbiota refers to this type of collection of microorganisms. However, in humans, there are trillions of such microorganisms living within the microbiome of the gastrointestinal tract, which is so significant that it is thought of as a ‘virtual organ’ within the body (Bull and Plummer, 2014).
The intestinal fungi regulate host homeostasis and play a key part in pathophysiological and physiological processes (Zhang et al, 2022). Within those trillions of microorganisms, there are bacteria, viruses, fungi and protozoa. The microbiome encodes more than three million genes, which produce many metabolites that act to replace the function of the host. This is why the microbiome itself has a direct link to affecting and influencing the host's fitness, phenotype and health (Bull and Plummer, 2014). Therefore, the microbiome has a direct influence on the development of diseases and cancers, and various other illnesses in a human being.
Hrncir (2022) explored the topic of gut microbiota dysbiosis—this is where the microbiota falls out of sync with the host and causes illness. It can be influenced by various factors. Hrncir (2022) found an increasing level of evidence pointing to gut microbiota playing a significant role in immune-mediated, metabolic and neurological diseases. The negative influences that could increase the likelihood of dysbiosis include environmental factors that can be changed such as unhealthy diet and also medication. Genes are unlikely to play a significant role due to being relatively stable.
Dysbiosis of the gut microbiota is a serious issue whereby the gut barrier is compromised, thus causing tissues and organs to be impacted directly by molecules from the diet, without the protection of the gut barrier. Due to this direct access, the immune system and metabolism can also be affected negatively. This causes a negative feedback loop, which accelerates and worsens the dysbiosis, such as in the case of liver disease, whereby the liver is unable to regulate the gut microbiota through bile acids and other modulating factors (Hrncir, 2022). However, some research in recent years have detected microbiota signatures of disease. Using this, early diagnosis can be made. These signatures could have diagnostic and prognostic value, and such value could be further enhanced through the detection of molecules in the blood, urine and faeces, which are also linked to the microbiota.
Further research on dysbiosis would need to place an emphasis on examining the microbiota-host interactions so that microbiota-based therapies for disease could be developed. This sort of research could help with the quest to alter the microbiota itself through the introduction of new strains that bring a benefit to the host, or through removing harmful strains successfully, which are known to be linked to disease. There is a possibility that the entire microbiota could be replaced through transplantation of faecal microbiota, or microbial metabolites could be introduced that either induce or block the production of certain metabolites (Hrncir, 2022).
The microbiota is considered by many research studies to affect the development of certain diseases, whereby any changes in the delicate composition of microorganisms can have a highly damaging effect on the host. Hrncir (2022) discusses the possibility that changes in gut microbiota may cause type one diabetes and Parkinson's, among other immunes disorders, as well as metabolic conditions and neurological diseases.
Probiotics
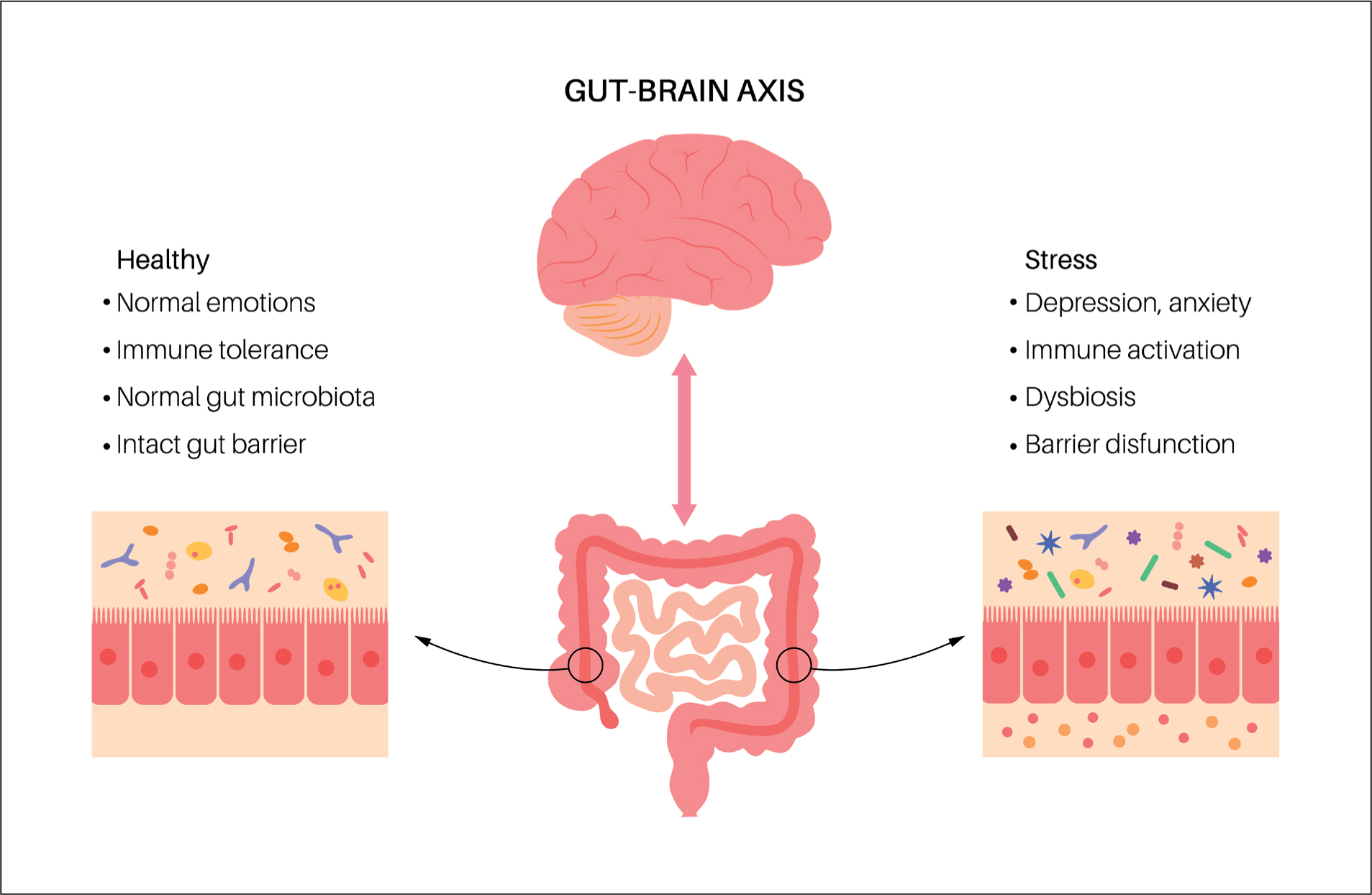
Clapp et al (2017) studied the gut-brain axis The researchers noted the strong bidirectional communication, which takes place between the central nervous system and the gut microbiota (Figure 1). The authors noted how dysbiosis can also have an impact on mental health, particularly being thought to play a role in anxiety and depression. The authors also noted the ability of probiotics to restore microbial balance; they explored whether this may mitigate the effect on anxiety and depression symptoms. The brain, gut and microbiome all link together, so where there is dysbiosis within the microbiome, there may be inflammation in the central nervous system, and these both have been linked as causes of mental illness (Clapp et al, 2017).
In their literature review, Clapp et al (2017) found that probiotics did appear to be linked to the mitigation of symptoms of anxiety and depression, and were observed to be similar in their effect to the conventional prescribed medications for such conditions. However, the research the authors reviewed was limited in that it failed to provide evidence of the link between Tumor Necrosis Factor Alpha, cytokines and other stressors, and the pathogenesis of anxiety and depressive disorders. Intestinal bacteria and the circulating cytokines linked to inflammation would need to be proven to be associated with the pathogenesis of such mental health disorders for the link to be confirmed.
Clapp et al (2017) also commented on the use of probiotics being questionable given these are not currently regulated by the FDA, and therefore, their benefits are not proven as a treatment. This includes other similar products such as kefir, yoghurt and sauerkraut. The authors found that research has confirmed a positive correlation between consuming such products and improvements in cognitive health and gastrointestinal health. There needs to be more robust evidence of the use of probiotics as therapy for mental health conditions; however, the overall benefit of probiotics is again called into question for many people as its effectiveness is weakened by comorbidities such as obesity, lifestyle, tobacco and alcohol use. Therefore, probiotics may not be particularly beneficial for health conditions when factoring the lifestyle factors and comorbidities.
However, Clapp et al (2017) went on to describe the imbalances and dysbiosis caused in the gut by mood-altering medications. This could benefit from the use of probiotics, as the dysbiosis caused by medications themselves can cause neurological imbalance. Therefore, it can be argued that probiotic could mitigate for this disturbance. The authors went on to question whether probiotics alone could fix such a disturbance of the mind, or whether they are needed in conjunction with mood stabilisers. However, this is highly unlikely.
Gagliardi et al (2018) studied the act of rebuilding the ecosystem of the gut following its experience of dysbiosis. The gut undergos significant fluctuations that are often accompanied by undesirable health outcomes. These fluctuations are influenced by lifestyle, stress, nutrition and antibiotics, and to rebalance the ecosystem of the gut would involve a personalised therapy that factors in all of these. Gagliardi et al (2018) noted that newer probiotic candidates have shown more promising results in counterbalancing the factors that lead to dysbiosis. There are trillions of microbes and many work with one another in the nature of gut dysbiosis; therefore, the authors discussed the need for a multi-probiotic approach. Other possible hopefuls in the tailored treatment of dysbiosis include faecal microbiota transplantation and bacterial consortium transplantation, predatory bacteria therapy, phage therapy and next generation probiotics.
Bolte et al (2021) examined long-term dietary patterns and their association with inflammation in the gut, notably looking at pro-inflammatory and anti-inflammatory features of the microbiome itself. The team found that there were shared responses of the gut microbiota to the diet among patients with Crohn's disease, ulcerative colitis and irritable bowel syndrome. They concluded that this may be relevant to other disease contexts where inflammation, gut microbial changes and nutrition are a common thread.
Bolte et al (2021) also found that people who had engaged in long-term diets rich in fruits, vegetables, legumes and nuts, who had an overall higher intake of plant foods over animal foods, with a preference for low-fat fermented dairy and fish, who avoided strong alcoholic drinks, processed high-fat meat and soft drinks, had the potential to avoid intestinal inflammation through their gut microbiome. In contrast to this, those with a diet unlike this were found in the literature to be at an increased risk of inflammatory bowel disease. Therefore, the authors concluded that gut microbiome can be modulated in order to produce therapeutic effects against inflammatory diseases of the gut. The evidence in the review by Bolte et al (2021) strongly favoured a plant-based diet.
Prebiotics
There are also a range of FDA-approved dietary supplements in use. One such supplement is HyFIBER by Nutrinovo. It is a liquid fibre supplement for bowel transit disorders. A clinician or dietitian would have to administer the product, which can be given as a liquid orally, or added to the enteral feed as prescribed. The product contains 12 g soluble fibre and has a citrusy taste. This product may help with gut motility and decrease the risk of constipation that many older patients suffer with, often a symptom of their condition or a side effect of their medication. Patients may be under nourished and benefit from this or other supplements but a dietitian's referral would be required, followed by their assessment and prescription, which would detail exactly what is required in terms of supplementing your patient's diet.
Conclusion
Overall, the gut microbiome is like an organ in itself—a virtual organ that inhabits the host and works in a bidirectional relationship between the gut and the brain. There are various hypotheses about the relevance of the gut microbiome in the pathogenesis of multiple diseases, and in the modulation of the microbiome in order to mitigate for disease. There is a strong argument about the inflammatory nature of many diseases and its link to gut microbiome dysbiosis. Fixing such a dysbiosis is now the focus of many pieces of research. Advice to patients should be on what is already known so far—it appears that a diet rich in legumes, fruit, nuts and vegetables, low in alcohol and processed foods and soft drinks, favours the better outcomes for modulation of gut dysbiosis.