Rheumatoid arthritis (RA) is a common chronic inflammatory autoimmune disease that primarily affects the synovial joints (Miao et al, 2013). It causes inflammation and thickening of the synovium, leading to pain and swelling, which can progress to erosion/damage to the cartilage and bone ends in the joint (Miao et al, 2013). As a progressive disease, it is associated with systemic complications (Firestein, 2003), socioeconomic costs (Merkesdal et al, 2005; Rkain et al, 2006) and consequences in all aspects of an individual's life (Eberhardt et al, 2007; Matcham et al, 2014). Despite improvements in the management of RA, it continues to be associated with increased morbidity, mortality and costs of care (Wolfe et al, 1994; Fautrel et al, 2007; Løppenthin et al, 2019). With more than 24 million people thought to have RA globally (Disease et al, 2016), it represents an often unrecognised challenge to health and social care services (Safiri et al, 2019). Although part of that challenge stems from the condition itself, RA is often accompanied by various comorbidities (additional condition(s) in the presence of a specific index condition of interest) that can affect different body systems (Gabriel, 2008; Espino-Lorenzo et al, 2013a; Jeong et al, 2017; Loppenthin et al, 2019). In addition to the specific effects of the comorbid condition, these may also aggravate the RA, which may cause functional decline, increase the costs of care and the person's risk of death (Gabriel and Michaud, 2009; Norton et al, 2013; Loppenthin et al, 2019).
Given the effects of comorbidities on people with RA, it is important to establish their prevalence to ensure appropriate health and social care services are provided. Several systematic reviews have assessed the prevalence of comorbidities among people with RA; however; these have limitations. Several have focused on specific comorbidities rather than a broad range of conditions—for example, cardiovascular events (Meune et al, 2010), coronary artery disease (Hansen et al, 2019), chronic obstructive pulmonary disease (Ma et al, 2019), diabetes (Boyer et al, 2011), lymphoma (Kaiser, 2008), fibromyalgia (Duffield et al, 2018), depression (Moll et al, 2011; Matcham et al, 2013) and hypertension (Boyer et al, 2011). Others have considered specific comorbidities within a single country—for example, cardiovascular disease in Latin America (Sarmiento-Monroy et al, 2012), depression in China (Fu et al, 2017) and depression in Iran (Jamshidi et al, 2019). As such, the authors undertook a systematic review to estimate the prevalence of a range of comorbidities that occur among adults with RA. In addition, we looked as to whether there was evidence of a difference in prevalence among different socioeconomic groups.
Aims
This systematic review aimed to estimate the prevalence rate of a range of comorbidities in adults with RA and to assess the moderating factors of country, age and a study's risk of bias.
Methods
Search
This systematic review was produced and reported following established guidance and standards (Moher et al, 2009; Higgins, 2019), having described our methods in a protocol registered on PROSPERO (registration number: CRD42019125556). This review has been reported in accordance with GRPP2 and the PRISMA 2020 Checklist (Staniszewska et al, 2017; Page et al, 2021). This protocol and the writing of this paper was supported by a member of the public in an advisory position, with the aim of gaining insights into important aspects that need to be included within the review from the public's perspective. This was achieved by meetings and draft copies of protocol and manuscript being reviewed by the public advisor. We searched EMBASE, PsycINFO and the Cochrane Library (Cochrane Database of Systematic Reviews) bibliographic databases from their inception to March 2019 (see supplementary information 1 for full search strategy). Results of the searches were exported to Endnote X9.0 software, with the results deduplicated.
Inclusion and exclusion criteria
Studies were included that reviewed point prevalence of 14 selected comorbidities (Box 1) among adults aged 18 years or older with RA. The 14 comorbidities were identified as being highly prevalent (>1%) in the general population (Barnett et al, 2012) and included in the quality and outcomes framework of UK general practice contracts (Forbes et al, 2017) and/or important study-specific diseases (Diederichs et al, 2011). Populations included could either have originated from: consecutively sampled RA patients (every subject that meets the inclusion criteria is selected); probability sampling of a broader representative RA population; an entire RA population or all adults with RA in a representative subpopulation of known size. Diagnosis of RA had to adhere to the 1987 American College of Radiology (ACR) or 2010 ACR/European Alliance of Associations for Rheumatology (EULAR) criteria (Arnett et al, 1988; Aletaha et al, 2010). The component comorbid disorders had to be derived from clinical records, a physician diagnosis, or a validated questionnaire or multimorbidity score/index. Studies had to report which selected conditions were used to define multimorbidity/comorbidity. Abstracts were only included if sufficient details were provided of their methods and results. Non-English language studies and those published earlier than 1987 were excluded (Arnett et al, 1988).
Box 1.Selected comorbid conditions
- Painful conditions (including fibromyalgia, osteoarthritis and back pain)
- Asthma
- Diabetes
- Thyroid disorders (including hypothyroid disease/hypothyroidism, hyperthyroid disease/hyperthyroidism/thyrotoxicosis)
- Hearing loss or deafness
- Chronic obstructive pulmonary disease (COPD)
- Depression
- Anxiety disorders (including, generalised anxiety disorder, social anxiety disorder, post-traumatic stress disorder (PTSD), panic disorder, obsessive-compulsive disorder, body dysmorphic disorder)
- Cancer or neoplasm
- Hypertension
- Coronary heart disease/ischaemic heart disease (including angina and myocardial infarction/heart attack)
- Stroke/transient ischaemic attack/cerebrovascular accident
- Atrial fibrillation
- Heart failure/heart insufficiency
Screening
Study selection occurred in two stages. First, titles and abstracts of studies were independently screened by two reviewers using pre-determined criteria. Second, manuscripts of papers identified as potential included studies at the first stage were retrieved and screened independently by two reviewers. Any disagreements were resolved by discussion or through arbitration by a third reviewer.
Data extraction and study quality assessment
Data were extracted using a pre-piloted form and included the following items: study/participant characteristics (sample, setting, etc) and outcome data (comorbidities reported, outcome measurement and time point for the data collected). See Supplementary file 1. Risk of bias was assessed for each study through the use of an adapted Hoy risk of bias assessment checklist for prevalence studies (Hoy et al, 2012). Each assessment resulted in a total score for each individual study, calculated from the nine individual questions. Higher scores represented higher levels of bias. Data extraction and risk of bias assessment were undertaken by a single reviewer and verified by a second reviewer. Any discrepancies were resolved through discussion or by a third reviewer.
We synthesised studies through a structured narrative synthesis with tabulation of the results of included studies, pooling relevant studies through meta-analysis using random effects models due to the likelihood of substantial heterogeneity (I-squared >50%). Meta-analyses were undertaken using the Project R Metafor package as part of the jamovi software (R Core Team 2018; The jamovi Project 2019). Heterogeneity was assessed using the I-squared statistic (Der Simonian-Laird), with possible causes assessed through meta-regression, using the moment method (Chen et al, 2012) and sensitivity analyses. Potential moderators were explored, including country, age and a study's risk of bias. This systematic review also assessed the effects of health inequalities on the prevalence of comorbidities associated with RA through indicators of socioeconomic status, such as education, income, and country's income (post-hoc subgroup).
Results
Our searches found 1008 records after duplicate removal, with 15 identified through checking of reference lists. Study selection identified a total of 33 studies (74 633 participants) for inclusion in the review and meta-analysis. A PRISMA flow diagram is given in Figure 1. The number of participants in the studies ranged from 40 to 29 260 (Chung et al, 2013; Klodzinski and Wislowska, 2018) (Table 1). Although the prevalence of all 14 comorbid conditions was assessed, the extent of evidence available differed. Hypertension, diabetes, stroke (including cerebrovascular accidents and transient ischaemic attack), chronic obstructive pulmonary disease, and coronary/ischaemic heart disease were all considered in six or more studies that included over 20 000 participants per condition. Cancer and depression were both assessed in 10 studies with over 6500 participants per condition. Most studies assessed the prevalence of comorbidities in high-income countries (n=24), with only eight in different medium- and low-income countries, and one study including high-, medium- and low-income countries (Table 1). Participants differed in mean age, ranging from 42 to 67 years (Emamifar and Jensen Hansen, 2018; El-Miedany and El Rasheed, 2002). Physician-based classifications using ACR criteria were used to diagnose RA in 30 (Table 1), with the remaining three studies using database/registry classification (Hashimoto et al, 2015; Emamifar and Jensen Hansen, 2018; Huang et al, 2018).
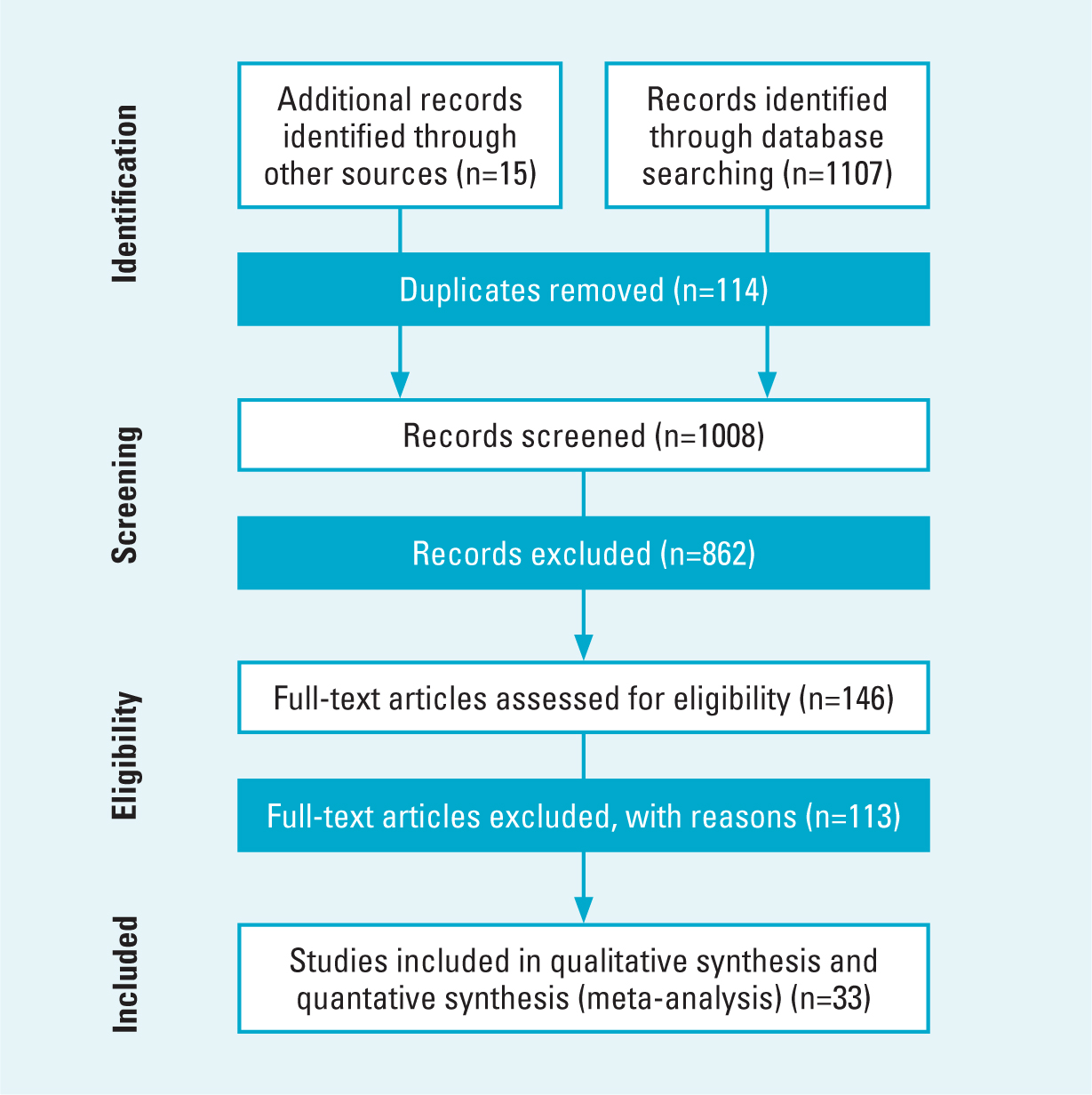
Table 1. Summary of included studies and their risk of bias rating
| Study | n | Mean age (SD) | Female (%) | ACR criteria | Country | Country income | Study design | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Abasalo (2008) | 789 | 61 (±13) | 72 | Physician | Spain | High | Cross-sectional | Low |
| Abda (2016) | 200 | 44.2 (±9.1) | 100 | Physician | Egypt | Low-middle | Cross-sectional | Moderate |
| Al-Herz (2016) | 835 | 50.6 (±12) | 62.3 | Physician | Kuwait | High | Cross-sectional | Low |
| Aurrecoechea (2017) | 140 | 51.4 (±14.4) | 50 | Physician | Spain | High | Cross-sectional | Low |
| Azuaga-Pinango (2018) | 130 | 58.6 (±12.9) | 79.2 | Physician | Spain | High | Cross-sectional | Low |
| Balsa (2019) | 200 | 58.5 (±12.4) | 79 | Physician | Spain | High | Cross-sectional | Low |
| Chandrashekara (2017) | 2982 | 48.98 (±12.64) | 84.4 | Physician | India | Low-middle | Cross-sectional | Low |
| Choi (2018) | 1050 | 56 (±12) | 83.1 | Physician | South Korea | High | Cross-sectional | Low |
| Chung (2013) | 29260 | 52.4 (±15.6) | 77 | Physician | Taiwan | High | Longitudinal cohort | Low |
| Dougados (2014) | 3920 | 56 (±13) | 81.7 | Physician | Multiple† | Mixed§ | Cross-sectional | Low |
| El-Mieldany (2002) | 80 | 41.93 (±8.43) | 88.7 | Physician | Egypt | Low-middle | Cross-sectional | Low |
| Emamifar (2018b) | 1035 | 67.1 (±14.5) | 63.4 | Dat/reg | Denmark | High | Longitudinal cohort | Low |
| Erb (2004) | 150 | 60.8 (±0.2) | 65.3 | Physician | UK | High | Cross-sectional | Low |
| Espino-Lorenzo (2013) | 114 | 53.4 (±11.7) | 80 | Physician | Spain | High | Cross-sectional | Low |
| Fan (2017) | 325 | 55.8 (±15.5) | 72.8 | Physician | France | High | Cross-sectional | Low |
| Gabriel (1999) | 450 | 64.1 (NR) | 74 | Physician | US | High | Longitudinal cohort | Low |
| Gist (2018) | 117 | 62.1 (±12.6) | 75.2 | Physician | Australia | High | Cross-sectional | Low |
| Haliloglu (2014) | 197 | 47.6 (NR) | 80.2 | Physician | Turkey | Low-middle | Cross-sectional | Moderate |
| Hashimoto (2015) | NR | 62.7 (±12.6) | 81.6 | Dat/reg | Japan | High | Longitudinal cohort | Low |
| Ho (2011) | 100 | 53.66 (±13.57) | 75 | Physician | Singapore | High | Cross-sectional | Low |
| Huang (2018) | 18267 | 53.6 (±13.9) | 78.4 | Dat/reg | Taiwan | High | Longitudinal cohort | Low |
| Jamshidi (2016) | 414 | 45 (±11.6) | 84.5 | Physician | Iran | Low-middle | Cross-sectional | Low |
| Klodzinski (2018) | 40 | 55.5 (±9.5) | 87.5 | Physician | Poland | High | Cross-sectional | Low |
| Naranjo (2008) | 4363 | 57 (±14) | 78 | Physician | Multiple‡ | High | Cross-sectional | Low |
| Panopoulos (2018) | 408 | 57.5 (±13.4) | 89 | Physician | Greece | High | Cross-sectional | Low |
| Panoulas (2007) | 400 | 61.56 (±12.02) | 73 | Physician | UK | High | Cross-sectional | Low |
| Przygodzka (2009) | 100 | 56 (±13) | 100 | Physician | Poland | High | Cross-sectional | Low |
| Pu (2018) | 161 | 47.14 (±12.04) | 76.4 | Physician | China | Low-middle | Cross-sectional | Low |
| Rahim (2018) | 192 | 51.98 (±11.22) | 88.4 | Physician | Malaysia | Low-middle | Cross-sectional | Low |
| Ranzolin (2009) | 270 | 55.0 (±12.4)* | 84 | Physician | Brazil | Low-middle | Cross-sectional | Low |
| Schau (2015) | 157 | 61 (±13) | 86 | Physician | Germany | High | Cross-sectional | Low |
| Sheen (2018) | 221 | NR | 70.6 | Physician | US | High | Cross-sectional | Low |
| Yamada (2011) | 7566 | 55.9 (±13.3) | 81.8 | Physician | Japan | High | Longitudinal cohort | Low |
Notes:
*RA patients only;
†Argentina, Austria, Egypt, France, Germany, Hungary, Italy, Japan, Korea, Morocco, Netherlands, Spain, Taiwan, UK, Uruguay, US and Venezuela;
‡Denmark, Finland, France, Germany, Ireland, Italy, the Netherlands, Poland, Serbia, Spain, Sweden, Turkey, UK, US and Argentina;
§three countries are low or middle and the rest are high);
Dat/reg=database or registry;
SD=standard deviation
Risk of bias
Using the Hoy risk of bias assessment checklist, 32 studies were rated to be at low risk of bias and one study was rated to be at medium risk of bias (Abda et al, 2016), as detailed in Table 2. The three criteria that the majority of studies failed to adequately address or not report were the use of an appropriate representative sample (n=28), the use of random sampling methods (n=27) and a response rate of less than 75% or which did not carry out a subgroup analysis comparing responders and non-responders (n=15). The remaining seven criteria were achieved by >80% of studies.
Table 2. Risk of bias assessment
| Abasolo et al (2008) | Abda et al (2016) | Al-Herz et al (2015) | Aurrecoechea et al (2017) | Azuaga-iñango et al (018) | Balsa et al (2019) | Chandrashekara et al (2017) | Choi et al (2018) | Chung et al (2013) | Dougados et al (2014) | El-Miedany et al (2002) | Emamifar et al (2018b) | Erb et al (2004) | Espino-Lorenzo et al (2013) | Fan et al (2017) | Gabriel et al (1999) | Gist et al (2018) | Haliloglu et al (2014) | Hashimoto et al (2015) | Ho et al (2011 | Huang et al (2018) | Jamshidi et al (2016) | Klodzinski et al (2018) | Naranjo et al (2008) | Panopoulos et al (2018) | Panoulas et al (2007) | Przygodzka et al (2009) | Pu et al (2018) | Rahim et al (2018) | Ranzolin et al (2009) | Schau et al (2015) | Sheen et al (2018) | Yamada et al (2011) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target population representative of national population | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sample representative of target population? | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Random selection or census undertaken | 0 | 1 | 1 | 1 | NR | NR | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Response rate ≥75% or no difference from nonresponders | NR | 1 | 1 | NR | NR | NR | NR | NR | 0 | NR | NR | 1 | NR | NR | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| Data collected directly from the subjects (not a proxy) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Acceptable case definition used in the study | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Reliabile, valid instrument for prevalence/incidence | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Same data collection mode used for all subjects | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Appropriate length of shortest prevalence/incidence period | 0 | 1 | 0 | 0 | NR | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | NR | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Appropriate numerator(s) and denominator(s) for prevalence/incidence | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Risk of study bias (low 1–4, moderate 5–7, high 8–11) | 2 | 5 | 3 | 4 | 4 | 2 | 3 | 3 | 1 | 4 | 2 | 3 | 3 | 3 | 2 | 3 | 3 | 4 | 3 | 2 | 1 | 4 | 4 | 3 | 4 | 3 | 3 | 3 | 3 | 4 | 3 | 3 | 3 |
Prevalence
When studies were pooled (Table 3 and supplement files number 1 Forest plots), anxiety disorders (62.1%, 95% CI: 43.6%; 80.6%) and depression (32.1%, 95% CI: 21.6%; 42.7%) were the most prevalent comorbidities among people with RA. Circulatory system conditions were also common. Hypertension (37.7%, 95% CI: 29.2%; 46.2%) and heart failure (10.9%, 95% CI: 2.5%; 19.3%) were particularly prevalent; however, coronary/ischaemic heart disease (6.9%, 95% CI: 0.3%; 13.5%), heart attacks/myocardial infarction (4.7%, 95% CI: 2.9%; 6.6%) and stroke (including CVA/TIA (2.3%, 95% CI: 1.9%; 2.7%) were also diagnosed. Studies reported the prevalence of several endocrine, nutritional and metabolic diseases, particularly thyroid disease (14.8%, 95% CI: 10.9%; 18.7%), diabetes (12.2%, 95% CI: 9.6%; 14.8%) and hypothyroid (2.9%, 95% CI: 0.0%; 6.1%). Respiratory conditions, including asthma (8.9%, 95% CI: 5.2%; 12.7%), COPD (4.7%, 95% CI: 3.4%; 6.1%), fibromyalgia (11.7%, 95% CI: 4.9%; 18.5%) and cancer (4.3%, 95% CI: 2.6%; 6.0%) were reported. All the meta-analyses were affected by considerable heterogeneity (I2 >75%) and should be interpreted cautiously (Higgins, 2019).
Table 3. Prevalence of comorbidities for people with rheumatoid arthritis
| Comorbidity | n | Prevalence | Heterogeneity Statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Lower 95% CI | Upper 95% CI | Z test (p value) | I2 | degrees of freedom | Q test (p value) | Risk of bias | ||
| Anxiety disorders | 1144 | 62.1% | 43.6% | 80.6% | < 0.001 | 98.3% | 5 | <0.001 | Low |
| Hypertension* | 57565 | 37.7% | 29.2% | 46.2% | < 0.001 | 99.8% | 14 | < 0.001 | Low |
| Depression* | 6700 | 32.1% | 21.6% | 42.7% | < 0.001 | 99.3% | 10 | < 0.001 | Low |
| Thyroid disease | 633 | 14.8% | 10.9% | 18.7% | < 0.001 | 90.2% | 5 | < 0.001 | Low |
| Diabetes* | 57897 | 12.2% | 9.6% | 14.8% | < 0.001 | 98.6% | 11 | < 0.001 | Low |
| Fibromyalgia | 1024 | 11.7% | 4.9% | 18.5% | < 0.001 | 96.0% | 4 | < 0.001 | Low |
| Heart failure | 942 | 10.9% | 2.5% | 19.3% | 0.011 | 96.8% | 3 | < 0.001 | Low |
| Asthma | 6226 | 8.9% | 5.2% | 12.7% | < 0.001 | 96.7% | 4 | < 0.001 | Low |
| Coronary/ischaemic heart disease | 20025 | 6.9% | 0.3% | 13.5% | 0.04 | 99.2% | 6 | < 0.001 | Low |
| Chronic obstructive pulmonary disease | 35385 | 4.7% | 3.4% | 6.1% | < 0.001 | 93.1% | 7 | < 0.001 | Low |
| Heart attack (myocardial infarction) | 39243 | 4.7% | 2.9% | 6.6% | < 0.001 | 97.9% | 5 | < 0.001 | Low |
| Cancer* | 8076 | 4.3% | 2.6% | 6.0% | < 0.001 | 93.9% | 10 | < 0.001 | Low |
| Hypothyroid | 19402 | 2.9% | 0.0% | 6.1% | 0.078 | 96.4% | 2 | < 0.001 | Low |
| Stroke† | 57618 | 2.3% | 1.9% | 2.7% | < 0.001 | 76.9% | 7 | <0.001 | Low |
Notes:
*Estimates from meta-regression and sensitivity analysis;
†including cerebrovascular disease and transient ischaemic attack
Meta-regression and sensitivity analysis
Given the heterogeneity, a sensitivity analysis and meta-regression was performed to assess the possible reasons. The analysis focused on the comorbidities of hypertension, depression, diabetes and cancer, as there were sufficient studies (ie ≥10 studies) (Higgins 2019) investigating the effects of population age, a country's income, and the study's risk of bias. Age explained a statistically significant amount of the variation in the prevalence of hypertension (R2=24%; p=0.019), depression (R2=80.5%; p<0.001) and cancer (R2=67%; p<0.001), although the effect varied between the different conditions. For hypertension and cancer, an increase in mean age by 1 year resulted in an increased prevalence of 2.3% (95% CI: 0.4%; 4.2%) and 0.5% (95% Cl: 0.2 %; 0.8%) respectively, while for depression it resulted in a decrease in prevalence of -4.8% (95% Cl: -5.8%; -3.9%). Unfortunately, for the mediating factors relating to health inequalities, only country income was able to be categorised. A country's income was important in influencing the prevalence of depression, with countries that had a low to moderate income having a prevalence rate of 40% (95% CI: 14.0%; 66.6%) compared with high-income countries. None of the factors provided a statistically significant explanation of the variation in the prevalence of diabetes. Further sensitivity analyses were conducted through focusing on different sub-sets of studies, but these had a limited effect on reducing heterogeneity, which remained substantial to considerable (I2 >73%).
Discussion
As a chronic condition, RA provides a continuing challenge to those with the condition, despite advances in approaches to its treatment. An important part of that challenge lies in the likelihood that people with RA will face other comorbid conditions, which may accentuate the symptoms of RA, worsen their general health and quality of life, and reduce their life expectancy. Developing appropriate services to prevent, identify and manage any associated multimorbidity necessitates recognition of the extent of the problem. Systematic reviews have assessed the prevalence of different comorbidities; however, these have focused on specific comorbidities and/or countries and used differing definitions of comorbid conditions (Kaiser 2008; Meune et al, 2010; Boyer et al, 2011; Moll et al, 2011; Sarmiento-Monroy et al, 2012; Matcham et al, 2013; Fu et al, 2017; Duffield et al, 2018; Hansen et al, 2019; Ma et al, 2019; Jamshidi et al, 2019). As a consequence, estimates of the prevalence of comorbidities in people with RA differ widely, causing uncertainty as to their impact and service requirements. This review endeavoured to provide some clarity, estimating the prevalence rate for a range of comorbidities in adults with RA using a standard definitions, clear criteria and rigorous methods.
When the evidence from 33 studies were pooled, it showed that anxiety and depression were particularly prevalent, with over 62% and 32% of people with RA with the conditions, respectively. These prevalence rates are notably higher than what is observed in the general population of 7.3% for anxiety (Baxter et al, 2013) and a one year prevalence rate of 7.2% for depression (Lim et al, 2018). Similarly, hypertension in people with RA was also prevalent compared to the general population (38% vs 31.1%) (Mills et al, 2020), as were other circulatory system conditions, such as heart failure (11% vs 0.8%) (Lippi and Sanchis-Gomar 2020) and coronary/ischaemic heart disease (7% vs 1.6%) (Khan et al, 2020). People with RA were commonly diagnosed with thyroid disease (15% vs 3.8%) (Garmendia Madariaga et al, 2014), diabetes (12% vs 9.3%) (Saeedi et al, 2019), fibromyalgia (12% vs 0.2 to 6.6%) (Marques et al, 2017) and asthma (9% vs 4.3%) (Loftus and Wise, 2016) compared to the general population. A person's age was an important factor in determining the prevalence of specific conditions, with older age increasing the prevalence of hypertension and cancer but reducing the prevalence of depression. A country's income also influenced the prevalence of depression, with those with lower incomes having a higher prevalence.
Where previous meta-analyses had been conducted, findings were often similar to ours, only differing where their focus was on specific population subgroups or where definitions of comorbidity varied. Although pooled estimates of the prevalence of depression in adults with RA varied in other reviews from 16.8% (Matcham et al, 2013) to 65.8% (Jamshidi et al, 2019), when using standard comparable measures, such as the HADS or PHQ-9 for moderate to severe depression, the prevalence was 34.2% (95% CI: 25%; 44%) and 38.8% (95% CI: 34%; 43%) respectively, similar to the prevalence from our meta-analysis (32%) (Matcham et al, 2013). We found that the prevalence of fibromyalgia in adults with RA was 12%, which was 9% lower than a previous estimate (21%, 95% CI: 17%; 25%) (Duffield et al, 2018). Further investigation revealed that a subgroup analysis (sample size >150 participants) in a previous meta-analysis (Duffield et al, 2018), which was similar to our evidence base, estimated a comparable prevalence for fibromyalgia of 14%. The prevalence of hypertension (38%), COPD (4.7%) and stroke (2.3%) were similar in our meta-analysis to that presented in previous meta-analyses (hypertension: 28%; COPD: 6.2%; stroke: 2.5%) (Sarmiento-Monroy et al, 2012; Matcham et al, 2013; Ma et al, 2019).
The influence of a person's age on the prevalence of depression in people with RA (ie increasing age resulting in a decreasing prevalence) was also reported in another meta-analysis (Matcham et al, 2013). It is evident that for conditions other than RA (eg cardiovascular disease) the relationship between age and depression is reversed, with the reason for the difference being unclear (Thielke et al, 2010). The influence of a country's income on the prevalence of depression, although not explicitly shown in previous meta-analyses, may partially explain, along with differences in diagnostic criteria, the considerable variation found in previous meta-analyses (range 0.04% to 66%) (Matcham et al, 2013; Jamshidi et al, 2019). Importantly, the authors identified that the prevalence of anxiety, depression, COPD, heart attack/MI, cancer and stroke (including CVA and TIA) were higher among people with RA than the general public, which reflected previous studies (Ma et al, 2019; Meune et al, 2010; Simon et al, 2015) (World Health Organization, 2017).
The different comorbidities have been shown to have a marked impact on people with RA, worsening their symptoms, their general health and their quality and length of life (Peterson et al, 2019; Machin et al, 2020). It is recognised that the provision of services should reflect the complex needs of the individuals and the different comorbidities they have, which may be numerous (National Institute for Health and Care Excellence, 2018; Daien et al, 2019;). Some national guidelines have acknowledged their importance (eg recommendation for regular monitoring and management of blood pressure) (Bombardier et al, 2012; Daien et al, 2019; Misra et al, 2008; National Institute for Health and Care Excellence (NICE), 2018). Given the diverse range of comorbid conditions and their potential impact on people with RA, there is a necessity for a comprehensive management programme that considers the effects of RA and the different comorbidities (Daien et al, 2019), offering a wide range of services that encompasses regular screening (NICE, 2018) and individual management plans (Scottish Intercollegiate Guidelines Network, 2011; Smolen et al, 2016; Daien et al, 2019; Ho et al, 2019).
Strengths and weaknesses of the review
Our systematic review had certain strengths, including:
- Protocol registration was carried out prior to commencing the systematic review
- A robust multi-database literature search was conducted
- Only studies that used ACR criteria (1987) or 2010 ACR/EULAR criteria were included
- Dual independent study selection, data extraction and a study's risk of bias were assessed using a pre-piloted processes and forms
- Support from a public advisor in developing the protocol, interpretation of findings and writing up of the study. The public advisor support also helped in identifying key areas within the paper that were difficult to interpret.
There were also certain limitations, which included:
- A limited set of 14 comorbidities were included in the review
- Selection of English language studies only
- Specific substantial heterogeneity was found for all analyses, which may result in reduced generalisability of these results
- Post-hoc subgroup analyses of the effects of country income
- Due to the limited number of studies, a focus on specific comorbidities in the meta-regression and subgroup analysis to explore heterogeneity.
Future research
It is evident that previous research has tended to focus on specific comorbidities, particularly around circulatory system conditions (eg. hypertension, heart disease and strokes), diabetes and COPD. Further research should be conducted into mental health conditions, thyroid disease, fibromyalgia, asthma and cancer, where the evidence base appears more limited. Heterogeneity identified in the meta-analyses highlights the considerable variation that our systematic review, and others, have uncovered. Further exploration of the causes of the variability should be undertaken to identify important moderators that require further investigation. This review selected specific comorbidities, but additional comorbidities should be included in an updated systematic review, and the effects of multi-morbidity considered.
Conclusion
People with RA are at risk of several multimorbid conditions, which may have consequences for the severity of the symptoms of their RA and their quality and length of life. Mental health conditions, specifically anxiety and depression, and hypertension appear most prevalent; however, limitations in the evidence base renders some findings uncertain. Given the importance of the comorbidities to people with RA, further research should clarify their extent more clearly to allow a judgement as to their consequences and the approaches needed to service development.
Key points
- Mental health and circulatory conditions are important comorbidities affecting people with rheumatoid arthritis (RA).
- Mean age is a moderating factor for the prevalence of hypertension, depression and cancer within adults with RA.
- Further research should consider the importance of other comorbidities and the effects of health inequalities.
- Further research should assess methods to best prevent, identify and manage these comor-bidities.
CPD reflective questions
- In regard to the most common comorbidities with rheumatoid arthritis, is there any additional information/knowledge you would need to develop support for patients with these conditions?
- What can you do within your own practice to support patients with rheumatoid arthritis and a comorbid health disorder?
- Within your own practice, do you carry out any regular screening for any of these conditions?


