Over the centuries, pandemics have annihilated human civilisation. During the last two millennia, the world has seen three pandemics of plagues, seven of cholera, several influenza pandemics and at least three epidemics and pandemics due to coronaviruses, with associated losses of millions of lives (Walsh, 2020). There has never been such interest, media coverage, availability of wealth of scientific data, advancement of knowledge and urgency in containing the pandemic as seen with COVID-19. At the time of writing, over 180 million people have contracted COVID-19 infection, and more than 4 million people have died of COVID-19 infection worldwide. Several million people are now experiencing ‘long COVID’-a persistence of symptoms from the acute phase of illness. Further, there are a large number of patients recovering from the complications of COVID infection following prolonged stay in the intensive care unit (ICU). Although the virus has not yet been contained, scientific advancements have certainly enabled a reduction in mortality.
The worldwide economic impact of the pandemic is immeasurable. The direct cost of preventing and treating COVID-19 infections alone is in excess of 11 trillion USD; another 10 trillion USD has been lost as indirect cost due to the loss of manpower during the past 18 months, and the cost is only rising (World Health Organization, 2020).
While knowledge regarding COVID-19 is evolving, a lot remains unknown. It is not yet clear whether the virus was transmitted zoonotically or if this was the result of an accident. It cannot be predicted how many waves of the pandemics will occur or how many variants of the virus will have to be dealt with, as well as the burden of viral loads with each variant. However, what has been confirmed is information about the mode of transmission, clinical presentation, disease course, effective treatment and prevention. The differences in transmissibility and virulence of the disease and outcomes in different age groups are intriguing. However, the pathophysiology of the disease and the microcirculatory changes that determine the prognosis are better understood now. This article focuses on the microcirculatory changes and immune coagulopathy caused by COVID-19 that are responsible for thromboembolic disease in arterial and venous circulation.
Coronaviruses
Coronaviruses belong to the Coronaviridae family and include four genera-alpha, beta, gamma and deltacoronaviruses. They are enveloped, positive-sense, single-stranded RNA viruses that infect a wide range of animals, including humans. Beta-coronaviruses include three highly pathogenic viruses, namely, severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV-2 (COVID-19), which cause severe pneumonia in humans (Masters and Perlman, 2013; Chams et al, 2020; Song et al, 2020; Yuki et al, 2020).
SARS-CoV-2 acts through angiotensin-converting enzyme 2 (ACE2) receptors that are widely distributed in the respiratory tract, renal tract and endothelium (Figure 1). The ACE2 receptors enable virus migration from the endothelium into the cells, where it can proliferate. In the endothelium, the virus stimulates the release of cytokines, such as tumour necrosis factor (TNF) and various interleukins (e.g. 1B, 1RA, 2, 6, 7), and chemokines, such as granulocyte-colony stimulating factor and platelet-derived growth factor (PDGF). Human coronaviruses cause seasonal respiratory diseases and gastroenteritis (Masters and Perlman, 2013; Chams et al, 2020; Song et al, 2020; Yuki et al, 2020).
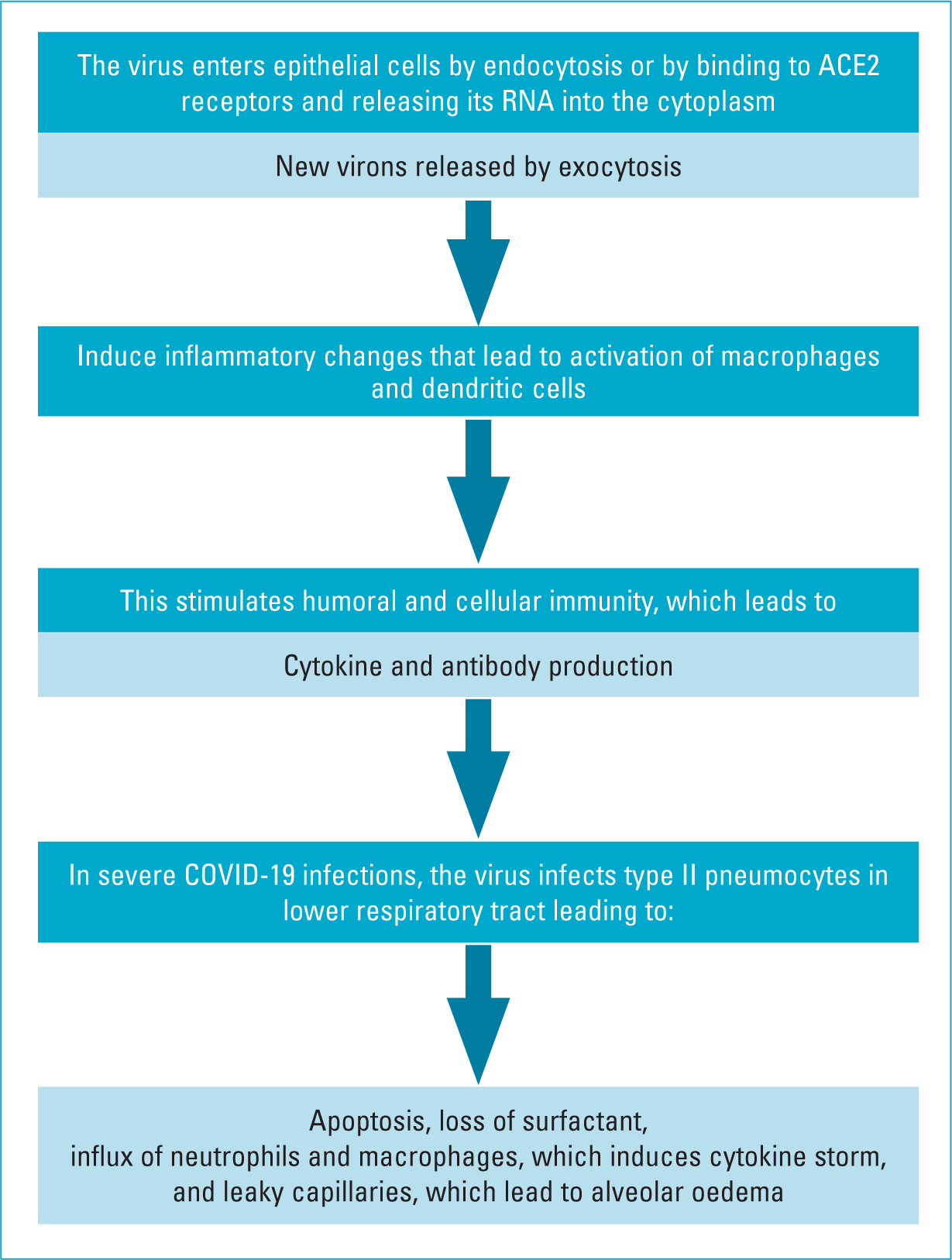 Figure 1. Pathogenesis of COVID-19
Figure 1. Pathogenesis of COVID-19
Although the pathogenesis of SARS-CoV-2 is not clearly understood, information regarding viral replication and pathogenesis can be extrapolated from the knowledge about other beta-coronaviruses (SARS-CoV and MERS-CoV) due to their similarities to SARS-CoV-2.
Direct viral injury
SARS-CoV-2 binds to epithelial cells in the oral and nasal cavities and can also migrate further down in the lower respiratory tract. It has been shown to infect primary ciliated cells in the conducting airway. Most of those infected will have a mild course limited to the upper and conducting airways (Chams et al, 2020). Like SARS-CoV, SARS-CoV-2 can infect alveolar type II pneumocyte cells. The virus is released in large numbers from infected type II pneumocytes and causes cell apoptosis. Type II pneumocyte cells (10–15% of total lung cells) produce surfactant, which reduces surface tension in alveolar walls. These cells are also responsible for maintaining the lung epithelium by epithelial regeneration (Fehr and Perlman, 2015). Therefore, as replicated SARS-CoV particles are released from the cell and move on to infect more type II pneumocytes, the resulting apoptosis eventually causes diffuse alveolar damage and impaired gas exchange, which is thought to lead to acute respiratory distress syndrome (ARDS). A similar mechanism is postulated for SARS-CoV-2 (Yuki et al, 2020).
Viral replication
As mentioned earlier, SARS-CoV-2 uses the ACE2 receptor for cell entry, like SARS-CoV. The receptor-binding domain (RBD) in the S protein of SARS-CoV-2 specifically recognises its host ACE2 receptor. Previous studies have shown that host susceptibility to SARS-CoV infection is mainly determined by the affinity between the host ACE2 receptor and the viral RBD in the early viral attachment phase. After cell entry, the viral RNA positive sense genome is released into the cell cytoplasm and undergoes translation and replication forming progeny genomes and sub-genomic mRNAs. Following further intracytoplasmic reaction, the pathogen gets transported to the plasma membrane and is exported out of the cell via exocytosis (Aykut et al, 2015; Grewal et al, 2021).
SARS-CoV and SARS-CoV-2 are involved with the renin-angiotensin-aldosterone system (RAAS) through ACE2, which functions as a receptor for both viruses and physiologically counters RAAS activation (Chams et al, 2020). There is some concern that the RAAS inhibitors used for medical management of hypertension may somehow contribute to poor outcomes through their effect on ACE2 (Chams et al, 2020). Conflicting data exists regarding the effect of RAAS inhibitors on levels and expression of ACE2 in various human tissues (Østergaard, 2021). The American College of Cardiology (ACC) advises that patients should continue taking RAAS inhibitors for conditions such as heart failure, hypertension and ischemic heart disease, and that, if COVID-19 occurs: ‘individualized treatment decisions should be made according to each patient's hemodynamic status and clinical presentation’ (Connors and Levy, 2020).
Microcirculatory changes are frequently observed in seriously ill patients in the intensive care unit (ICU) and are related to the severity of the disease. The changes usually observed are an increase in these microcirculatory parameters and RBC velocity as a response to hypoxia. These have been documented in patients with COVID-19 infection admitted to ICU (Hawiger et al, 2015; Martini, 2020; Scavone et al, 2020). The microcirculatory parameters usually monitored are:
- Functional capillary density (FCD)
- Proportion of perfused vessels (PPV)
- Red blood cell velocity (RBCv) and total vessel density (TVD).
Cytokine storm and endothelial dysfunction are responsible for the microvascular damage in patients (Figure 2). As discussed previously, there is a state of elevated cytokines and chemokines in patients with COVID-19 as an augmented immune response observed in the later phases of the disease. Consequently, there is severe endothelial dysfunction that leads to multiorgan failure. Cytokine storm leads to activation of platelets, neutrophils, monocytes and macrophages. These cells adhere to the adhesion molecules (ICAM and VICAM) exposed by damage to the endothelial cells. The mitochondria in the endothelial cells release oxygen-derived free radicals, with a reduction in endothelial NO synthase (eNOS) activity and nitric oxide (NO) levels. NO is vital in endothelial microhemodynamic regulatory activity. The continuing damage in the alveoli with interstitial oedema progresses to ARDS and systemic hypoxia. Hypoxia-inducible factor 1 (HIF1) may subsequently trigger the release of vascular endothelial growth factor (VEGF), leading to massive interstitial lung oedema.
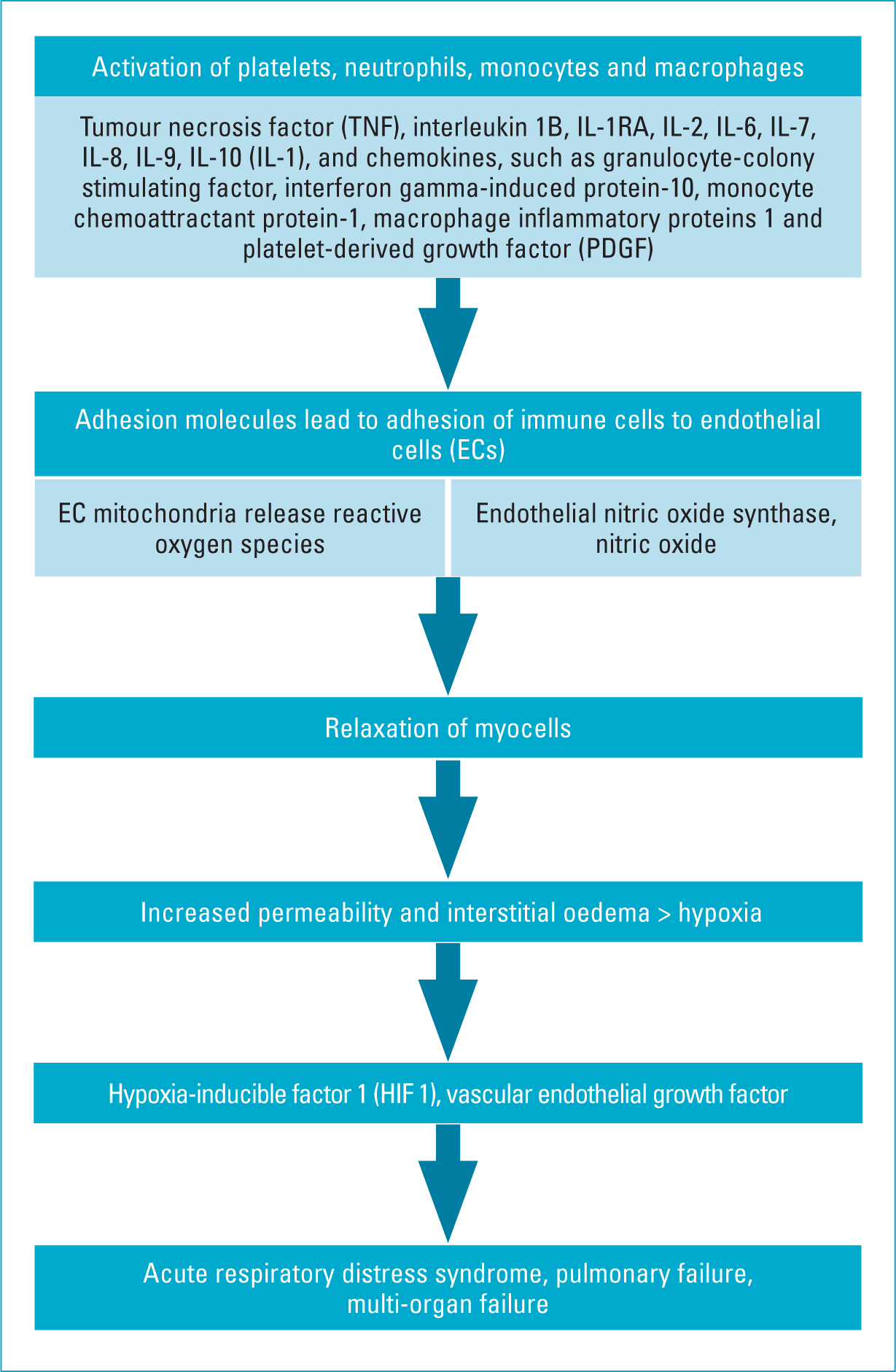 Figure 2. Phenomenon of cytokine storm in COVID-19
Figure 2. Phenomenon of cytokine storm in COVID-19
Endothelial cells regulate haemostasis via the production of the von Willebrand factor (VWF), tissue factor (TF) and plasminogen activator inhibitor type 1 (PAI-1) (Connors and Levy, 2021). VWF binds platelets to collagen components exposed following destruction of endothelial cells (Tan et al, 2021). TF, together with factor VII, activates factors IX and X. PAI-1 regulates fibrinolysis by inhibiting tissue plasminogen activator. Endothelial cells also activate protein C via thrombomodulin and endothelial protein C receptor, which inhibits factor V, factor VIII and PAI-1. In those with severe COVID-19, a hypercoagulable status develops, charaterised by an elevated level of fibrinogen, D-dimer, protein C, IgG anti-cardiolipin antibodies (ACA), anti-beta2-glycoprotein I (anti-2-GPI), factor VIII and VWF. There is also a reduction in antithrombin III and protein S antigen. The resulting prothrombotic effects stimulate further adhesion of leukocytes and platelets to the vascular endothelium, causing a further increase in endothelial dysfunction with vascular micro-thrombosis, capillary plugging and greater impairment of capillary flow. Thus, in COVID-19, the cytokine storm in the late phase of the infection may cause microvascular dysfunction starting with endothelial cell injury (Figure 3).
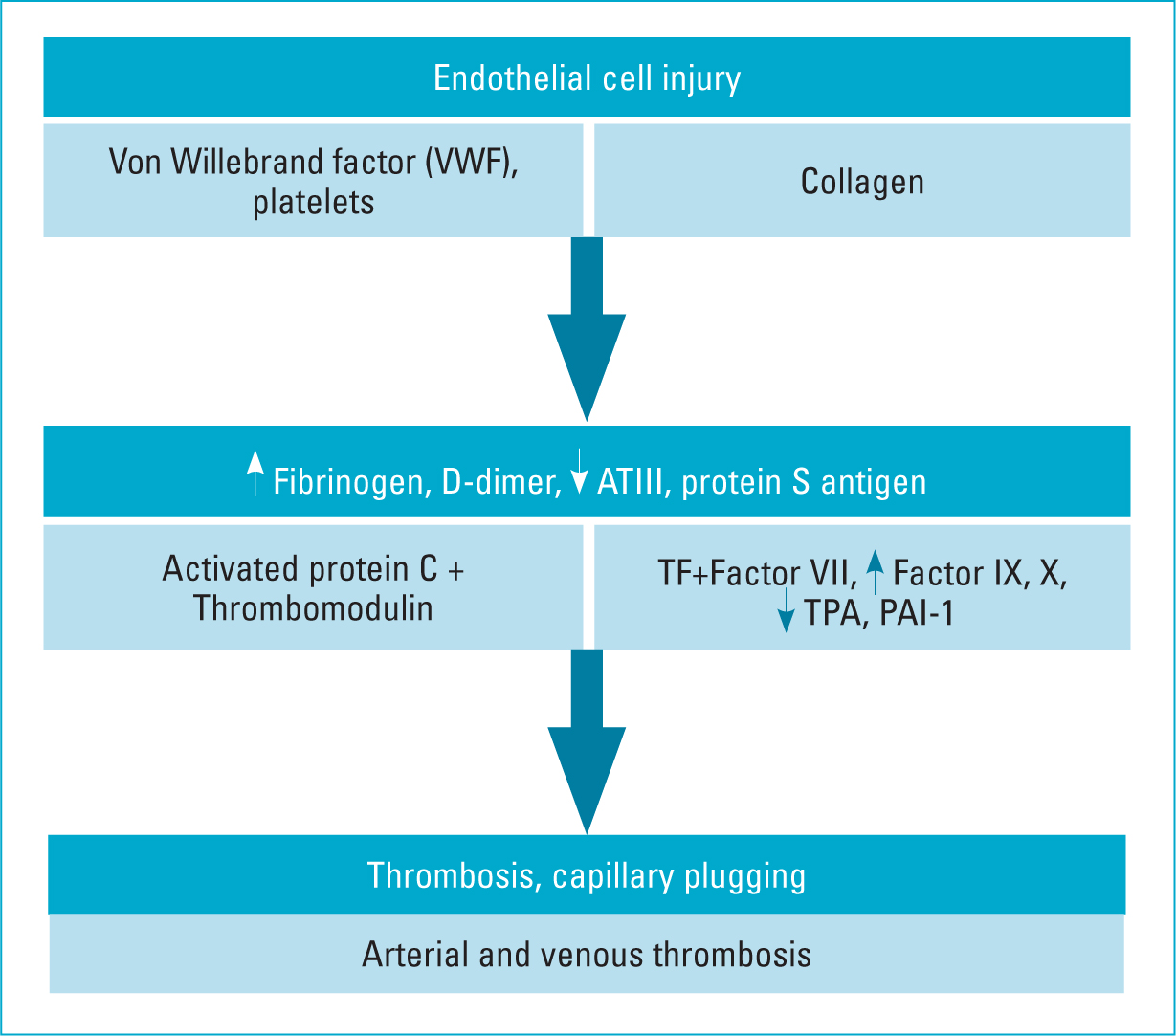 Figure 3. Mechanism of activation of thrombosis in COVID-19
Figure 3. Mechanism of activation of thrombosis in COVID-19
The main outcomes of these events are a maldistribution of organ perfusion, endothelial barrier leak and a prothrombotic state. Further, deformation of red blood cells and leukocytes may induce capillary plugging and capillary thrombosis, with microcirculatory stasis and increased tissue hypoxia. The clinical findings related to the microvascular dysfunction are ARDS and multiorgan failure. Blood hypercoagulability with disseminated microvascular thrombosis may be also sustained by a diffuse and massive endothelial injury. The cytokine storm then plays a key role in the progression of the disease towards a critical stage and could be considered as a prognostic indicator of fatal events in COVID-19 (Violi et al, 2021).
Levels of C-reactive protein and the erythrocyte sedmintation rate generally elevated in COVID-19, but procalcitonin levels are usually normal. A high procalcitonin level may indicate a bacterial co-infection. Studies have shown that elevated levels of lactate dehydrogenase, serum ferritin, IL-6 and high-sensitivity cardiac troponin I were all associated with worsening illness and higher mortality (Chams et al, 2020).
Bedside microvascular evaluation remains a problem. Most available instruments do not have good diagnostic accuracy. Laser Doppler techniques, nailfold capillaroscopy, transcutaneous oxygen pressure measurement (Tc PO2) and near-infrared spectroscopy have been used to study microcirculation, but hand-held video microscopy (HVM) used in the ICU in critically ill patients to observe the sublingual microcirculation has a better track record for bedside assessment (Aykut et al, 2020; Martini, 2020; Grewal et al, 2021). HVM devices, equipped with imaging techniques, enable real-time microvascular monitoring in these patients. Microcirculatory changes have been noted in advance of changes in systemic hemodynamic parameters in many clinical settings that are associated with cardiovascular compromise. HVM also allows monitoring patient's progress and response to treatment. Together with HVM, monitoring biomarkers for inflammation, tissue perfusion, coagulation and transport of oxygen to the tissues may provide a better indication of a patient's progress than HVM alone. Recently, a new diagnostic tool known as flow-mediated skin fluorescence (FMSF) has been used for noninvasive diagnostics and monitoring of the microcirculation (Martini, 2020). FMSF measures changes in NADH fluorescence intensity as a response to blocking and releasing blood flow in the forearm, as a function of time. Flow motion response to hypoxia could be useful for determining the severity of COVID-19 symptoms, particularly considering the recent observation that hypoxaemia is independently associated with COVID-19-related in-hospital mortality.
Clinical manifestation of thrombosis
Several reports have been published regarding thrombotic complications related to COVID-19 infection; undoubtedly, the rise in thrombotic complications is real (Gebicki et al, 2020; Goldman et al, 2020; Connors and Levy, 2020; Malas et al, 2020; Singhal, 2020; Violi et al, 2020; Jenner and Gorog, 2021; Tan et al, 2021). However, most thrombotic events are related to the severity of the disease in the ICU setting. Further, these thrombotic events can affect both arterial as well as venous circulation. A detailed account of the frequency of thrombosis in different settings and in different vascular territories derived from at least two reviews and meta-analyses and few other case reports is summarised here.
Evidence for microthrombosis
Clinicians and scientists have been examining the development of microthrombi in patients with COVID-19 (Ackermann et al, 2020; Lin et al, 2020). Surprisingly, a widespread thrombotic process has not been confirmed in most pathology specimens examined. There is only one report that has confirmed a few thrombi in skin biopsies from three patients who presented with a purpuric rash (Colmenero et al, 2020). There have been no published reports demonstrating widespread multiorgan thrombi in COVID-19 patients. Other rare pathologic findings that have been reported in COVID-19 patients include viral inclusions, oedema, intra-alveolar fibrin, and endotheliitis (Varga et al, 2020). However, there is no evidence that any of these pathologic findings are pathognomonic of COVID-19.
Arterial and venous thromboembolism in COVID-19
Tan et al (2021) performed a systematic review and meta-analysis of 102 studies comprising 64 503 patients to provide pooled estimates of venous and arterial thromboembolism rates and mortality risk. Malas et al (202) reported composite outcomes of venous thromboembolism in their review and meta-analysis of 16 studies. There was a high heterogeneity in these studies related to bias, population, settings and prevalence of venous thromboembolism. Despite this, the available evidence indicates that COVID-19 poses a significant risk of venous and arterial thromboembolism, and prevention strategies could reduce COVID-19 mortality.
Thrombotic complications of COVID 19 in the community
The incidence of a thrombotic event in patients who test positive for COVID-19 in the community and remain asymptomatic or develop mild symptoms is very low (Martini, 2020).
Thrombotic complications of COVID-19 among hospitalised patients and in the ICU setting
There is wealth of data from multiple perspective and retrospective studies from around the world regarding the incidence of thrombotic complications in hospitalised patients. In a large cohort of 2748 patients hospitalised due to COVID-19, 86 patients (3.1%) were found to develop venous thromboembolism, including deep vein thrombosis (DVT) (1.5%) and pulmonary embolism (1.3%), including seven cases of arterial thromboses (0.3%) (Martini, 2020). The incidence of thrombosis in patients with COVID-19 requiring care in the ICU appears to be higher than that in non-ICU hospitalised patients.
Thrombotic complications of COVID-19 following discharge from hospital
The risk of thrombosis is highest in patients with severe COVID-19, and this risk continues in the community after discharge from hospital, suggesting the need for extended thromboprophylaxis in such patients.
Conclusion
The COVID-19 pandemic has taken the world by great surprise and has led the scientific community to act at speeds never seen before.
Despite all the available information, the way the virus interacts with the cells to cause the disease and propagate to damage multiple organs with serious outcome remains unclear. However, the changes seen in microcirculation correlate with the severity of the disease. With the use of HVM and FMSF, it is possible to monitor the response to treatment and patient progress, as well as predict the outcome in affected individuals. The association of COVID-19 with venous thrombotic complications is undisputed, with the severity of COVID-19 being directly proportional to the risk of thrombotic complications, although the prevalence of arterial thrombotic events is still not well understood. It is important to ensure thromboprophylaxis in patients who needed ICU care for COVID-19 even after discharge to the community.
KEY POINTS
- The severity of the COVID-19 infection is related to the cytokine storm, hypoxic insult and activation of thrombosis
- Patients with serious COVID-19 infections are mostly seen in the intensive care setting
- Assessment of sublingual microcirculatory changes enables monitoring response to treatment and predict prognosis
- There is evidence to suggest an increase in the incidence of venous and arterial thrombosis in patients with severe infection in the intensive care setting
CPD REFLECTIVE QUESTIONS
- What are the extrinsic and intrinsic factors that determine the severity of COVID-19 infection?
- What are the clinical manifestations of microvascular thrombosis in COVID-19?
- What thrombopropylactic measures can be taken to ensure that patients who received ICU care for COVID-19 do not develop thrombotic complications?


