The presence of lymphoedema is one example of a long-term condition that requires self-management, alongside the support of significant others and healthcare professionals. However, this area becomes complicated when this is accompanied by other long-term conditions, such as heart failure (HF), with compression therapy (CT) being contraindicated in some cases (International Lymphoedema Framework (ILF), 2006a; Moffatt et al, 2019). A recent international study suggested that the average age for those affected by lymphoedema are those over 55 years, with more woman affected than men (Mercier et al, 2019). This is in comparison to the average age of those affected by HF, which is 72 years, with men being more commonly affected than women (National Institute for Health and Care Excellence (NICE), 2018). Statistically, 920 000 people are affected by HF within the UK, with lymphoedema estimated to affect between 3:99 up to 28.86 per 1000 of the UK population (Moffatt et al, 2016; NICE, 2018). It has been suggested that the presence of HF may negate the application of CT, due to the potential impact this may have upon the person's physical health (Moffatt et al, 2019a; 2019b). CT comes in a variety of formats, such as compression hosiery (CH) and bandages, or varying levels of pressure being delivered in millimetres of mercury (mmhg) (ILF, 2006a). The possible connection between the effects of CT, and the movement of fluid within a compromised cardiac system, has led to a variety of approaches in the management of oedema (Andriessen, 2017). The aim of the scoping review (SR) is to identify the current level of evidence and guidance within the application of compression therapy in those diagnosed with heart failure and/or chronic oedema/lymphoedema. The outcomes of the SR will be presented as a narrative synthesis of the three challenges that are often faced when considering CT in those with HF and/or chronic oedema/lymphoedema.
Method
Scoping review
The selection and application of SRs has gained popularity within nursing and healthcare, due to the speed it can be undertaken (Bradbury-Jones et al, 2019). However, it is not the same as a systematic review, in terms of the detail, analysis or the presence of a systematic approach in the gathering of evidence (Sucharew et al, 2019). Its broad approach is intended to establish the need for further research, such as systematic reviews, or to inform policy creation or assist in the mapping of key concepts within a subject area (Peters et al, 2020). Despite SRs not requiring the same amount of rigour as a systematic review, an SR still employs several similar processes (Bettany-Saltikov and McSherry, 2016). It has been suggested there are over 39 type of SRs, with each having its own merits; however, for the purposes of this SR, the framework offered by Peters (2020) (Table 1) has been employed.
Table 1. Scoping review framework
| Stages | Description |
|---|---|
| 1 | Defining and aligning the objective(s) and question(s) |
| 2 | Developing and aligning the inclusion criteria with the objective(s) and question(s) |
| 3 | Describing the planned approach to evidence searching, selection, data extraction and presentation of the evidence |
| 4 | Searching for the evidence |
| 5 | Selecting the evidence |
| 6 | Extracting the evidence |
| 7 | Analysis of the evidence |
| 8 | Presentation of the results |
| 9 | Summarising the evidence in relation to the purpose of the review, making conclusions and noting any implications of the findings |
Identification of articles and guidance
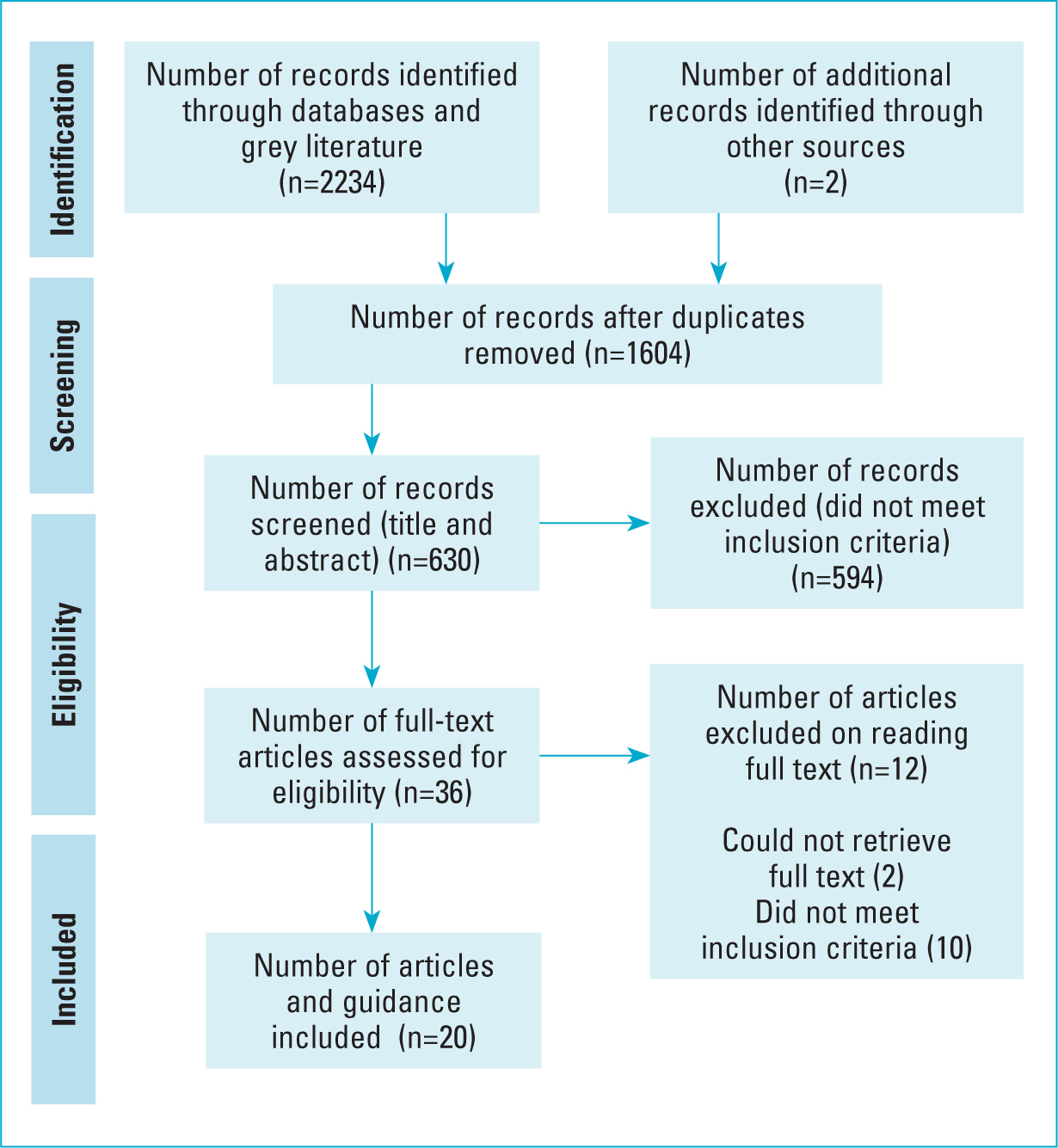
The initial review led to the devising of keywords within the population intervention comparison and outcome (PICO) framework, coupled with the use of mesh terms, and the devising of a search strategy, as shown in the examples in Table 2 (Bettany-Saltikov and McSherry, 2016; Aveyard, 2019). These keywords were combined with the use of Boolean terms in multiple databases, which included CINAHL, MEDLINE, ProQuest and Cochrane, alongside searches via Google Scholar to obtain a sample of grey literature (Bettany-Saltikov and McSherry, 2016). In stage 5 (Table 1), a two-stage approach was applied: 1) a title and abstract review and 2) full-text reviews, which were undertaken against the inclusion and exclusion criteria (Bettany-Saltikov and McSherry, 2016) (Table 3). The results of the study were taken and presented within a Preferred Reporting and Identification for Systematic Review framework (Scoping Review Extension PRISMA-ScR) (Peter et al, 2020) (Figure 1).
Table 2. Search terms (combined with ‘or’ and ‘and’)
| 1 | ‘man*’, ‘women*’ |
| 2 | ‘compression therapy,’ ‘compression hosiery’ |
| 3 | ‘no compression’, ‘no compression therapy’ |
| 4 | ‘homeostasis’, ‘cardiac’ |
| 5 | ‘primary research’, ‘guidance’ |
| 6 | ‘heart failure’, ‘chronic oedema’ |
Table 3. Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| 1. Peer-reviewed literature publications (primary and secondary research), grey literature, national guidelines and consensus documents | 1. Non-peer-reviewed literature (primary and secondary research), commentary articles or material that is not national guidelines or consensus documents |
| 2. Participants aged over 18 years diagnosed with the presence of heart failure and/or chronic oedema/lymphoedema | 2. Literature focusing upon those under the age of 18 years, without the presence of heart failure and/or chronic oedema/lymphoedema |
| 3. Partcipants undergoing or have received compression therapy in response to oedema | 3. Partcipants not undergoing or have received compression therapy in response to oedema |
| 4. English language paper with no date restrictions | 4. Non-English language papers |
Data extraction and analysis
Extraction of the data followed the approach taken by Peters et al (2020) (Table 1), which led to a review of the included evidence sources, with themes identified across these. Narrative synthesis was then employed within the SR, with an acknowledgement that other approaches could have been employed (Peters et al, 2020). However, the decision to implement a narrative synthesis was based on its ability to bring together sources that are varied in their presentation and/or results, but in which a story is present that would not otherwise be identified (Popay et al, 2006). The results are presented as themes, due to the similarities found across the evidence base, which, in this paper, are classed as ‘challenges’ (Popay et al, 2006).
Findings: overview
Some 20 pieces of evidence were retrieved from varying backgrounds and locations on the application of CT in those diagnosed with HF. In terms of location, the evidence retrieved included studies conducted in the US (one), Brussels (two), England (one) Europe (two), Israel (four), Japan (one), Northern Ireland (one), Poland (one), Scotland (one) Sweden (one) and the UK as a whole (five). Research designs or approaches within the retrieved evidence included a systematic review, literature reviews (two), guidelines/consensus documents (10) and comparative (six) and retrospective (one) studies. Three common challenges were identified across the 20 pieces of evidence and are discussed below.
Challenge 1: heart failure and lymphoedema
The variety of evidence presented within the SR indicate the challenge of managing chronic oedema within those diagnosed with HF. Despite there being a variety of studies, not all focused upon or included participants diagnosed with chronic oedema/lymphoedema. However, these studies do not always consider alternative causes of oedema formation, such as chronic oedema/lymphoedema, or even the presence of both conditions. This is despite a study by Urbanek et al (2020), which suggests up to 42% of those diagnosed with HF suffer with bilateral ankle oedema, compared to 22% who did not have a HF diagnosis. This is also set against figures that suggest up to 7% of those diagnosed with lymphoedema have elevated levels of B-type natriuretic peptide (BNP) that may indicate a HF diagnosis (Urbanek et al, 2020). This may indicate an underestimate of the presence of both conditions within these patient groups. A recent national study suggests HF is one of the six independent factors associated with the development of the chronic oedema, representing 27.3% of the sample population (Moffatt et al, 2019b).
The guideline offered by NICE (2018) suggest a variety of investigations relating to HF, such as a BNP blood test. An echocardiogram is required to determine the diagnosis, staging and even stability of the condition. However, even if the HF is stable, a review by Andriessen et al (2017) noted concerns about the use of CT, even with groups of patients classed as stable. This was due to the potential of fluid displacement from peripheral, such as the lower limbs, to central locations, such as the heart (Andriessen et al, 2017). A study by Nose (2010) applied intermittent pneumatic compression (ICT) in those with HF, which led to an increase in the venous flow in the soleal and popliteal vein. However, a study by Rabe et al (2020) identified that only a fraction of the blood displaced from the lower limbs reached the heart when CH (Class 1 and 2) was applied.
Despite the different CT applied, a more recent study by Nose (2020) extends their previous work with IPC. Nose (2020) identified a statistical significance in the amount of fluid that returned to the left side of the heart when IPC was applied, but noted that this increase was reversed after 30 minutes of IPC being stopped, with limited effects upon left-sided function of the heart (Nose et al, 2020). These short increases in cardiac pre-load were also noted by Rabe et al (2020), but they were asymptomatic within these patients. In Urbanek et al's (2020) review, these changes were also seen to be reversed within 10 minutes following the application of CT.
The evidence in this category suggests that HF and chronic oedema/lymphoedema may not always be separate. The assumption that CT applied to those with HF will lead to lasting physical changes may not always apply and may require further clarity.
Challenge 2: guidance and guidelines
When reviewing guidance, a variety of sources were located from different institutions, from public bodies to charitable organisations. Each item of guidance had its own aim, focus and approach. When considering the guidance offered by (NICE, 2018) and the Scottish Intercollegiate Guidelines Network (2016) on HF, there is no mention of the use of CT, or it being a contraindication in those diagnosed with HF. There is also no mention of the potential impact of other long-term conditions that may be present, such as chronic oedema/lymphoedema. In comparison, the Clinical Resource Efficiency Support Team (CREST) (2008) guidelines for lymphoedema consider uncontrolled HF as an absolute contraindication for the use of CT. The stance adopted by CREST (2008) complements that taken by the ILF (2006a; 2006b) in the production of the consensus guidance for the management of lymphoedema and the use of CH, in which the only contraindication is in instances where HF is uncontrolled. This perspective is also present within the ILF's documentation on palliative care and compression bandaging (CB), with CT only restricted if the HF is unstable, or classed as acute congestive heart failure (ILF, 2010; 2012).
In more general guidance offered by Wounds UK (2019; 2021), in which chronic oedema may be present alongside venous leg ulcers, the diagnosis of HF has led to a cautionary approach being taken, such as CB applied to one leg at a time if asymptomatic (Wounds UK, 2019). The consensus document by Rabe et al (2020) suggests those with a stage 1–2 NYHA may receive compression if their HF is classed as stable, due to the presence of short increases in cardiac pre-load that were asymptomatic. Rabe et al (2020) only cautions against the use of CT in those with NYHA stage 3–4, but this is contextualised within clinical need. These varying perspectives regarding the management of HF and the use of CT can cause confusion and uncertainty, especially when set against other sources of evidence present in this area. This may lead to decisions and even guidance being based upon theoretical concerns about CT within these patient groups, rather than wider considerations or clinical evidence (Andriessen, 2017).
Challenge 3: compression therapy and heart failure
The varying guidance across both patient groups leads to consideration of the evidence surrounding HF and CT, even in the absence of participants being diagnosed with chronic oedema/lymphoedema. The evidence retrieved considered four of the common approaches to CT: 1) CB; 2) CH; 3) manual lymphatic drainage (MLD); and 4) IPC.
The retrospective study by Attaran (2020) applied both CB, CH and a version of IPC across 95 patients with a diagnosis of HF (diastolic: 72.6%; systolic: 11.6%; mixed: 15.8%). Up to 32.6% of these patients were also diagnosed with lymphoedema. The majority wore CB over an average of 310 days, with only seven patients decompensating (unstable) between 49–1647 days, which represented 7.3% of those wearing CT. This potential complication (decompensation) was 10–13% lower in the CT group when compared to the average percentage of 17–20.7% that occurred without CT in the study's geographical area (Attaran, 2020).
The single-centre comparative study by Papismadov (2019) investigated postural hypotension (PH) and its prevention through the use of CB. A HF diagnosis represented 41% of the 100 people who were to subject to CB, with pressure levels reaching 40 mmhg at the ankle (Papismadov, 2019). The study's findings suggest there were no ill effects identified in those who received CB and were diagnosed with HF, with a relative risk reduction of 47% for PH.

The final comparative study by Gorelik (2009) focused upon those with HF (NYHA 2: 51%; NYHA 3–4: 49%), in which 49 people received CB (40 mmhg at the ankle) following detection of PH. The findings suggest that PH was prevented in 21 out of the 49 (43%) participants, with no ill effects noted and symptoms of dizziness and/or palpations being the same in those who did not receive CB (Gorelik, 2009).
Even within the single-group study by Kierenkegaard (1992), the use of CH (18 mmhg) in 47 patients with decompensated (unstable) HF illustrated that there were limited ill effects. When we consider the use of IPC to that of CB and CH, the evaluation studies by Bickel (2014), Moady et al (2019) and Nose et al (2010), with sample sizes of between 14–19 patients, NYHA bandings of 2–4, and applied compression levels of between 50–130 mmhg for 40–60 minutes. The findings from these studies (Nose et al, 2010; Bickel, 2014; Moadey et al, 2019) collectively established that there was an absence of ill effects felt by those subjected to the IPC, which was also noted in the use of MLD (Leduc, 2019). Leduc's study (2019) included nine patients with a stable diagnosis of 3–4 NYHA, who received 15 minutes of MLD, which led to circumferential reductions and no ill effects.
The findings of these studies indicate the potential application of a variety of CT forms to those with HF. This complements the review by Urbanek (2020), who stated that CT is reasonable and safe in most people diagnosed with NYHA stages 1–2 who are stable; however, the researcher advocated a multidisciplinary team approach to decision-making around this. Andriessen et al (2017) stated that the only true contraindications to use of CT are critical ischaemia, such as an ankle brachial pressure index score of below 0.5, and the presence of pulmonary oedema. However, there is also a limitation on the generalisability of these studies, due to the limited inclusion criteria or the absence of those diagnosed with chronic oedema/lymphoedema.
Strengths and limitations
The strengths of this study include its capacity to bring together a broad variety of sources across HF literature and to consider its relevance to chronic oedema/lymphoedema. This led to the identification of numerous sources of evidence that consider compression therapy within HF.
However, one of the limitations of this approach is that any literature found may not always be within the sphere of lymphoedema, such as patients diagnosed with heart failure only, but may receive compression therapy. However, this study is an SR, with its aim of identifying evidence more broadly, rather than being confined to one area (Peters, et al, 2020). Another limitation compared to a systematic review is the absence of appraising the chosen evidence; however, this is not the intention of this SR, and is not present in a number of SR designs (Peters, et al, 2020).
There are also noted limitations within the studies investigated, including varying or unclear reporting of cardiac status or staging, with not all participants having a diagnosis of chronic oedema/lymphoedema, and with not all stating the exact level of compression applied, such as Attaran et al (2020) or Papismadov et al (2019). However, the majority of retrievals included did use the New York Heart Association (NYHA) heart failure classifications/staging. Overall, the study presents the current landscape of the evidence within HF literature related to the use of compression therapy and its potential within chronic oedema/lymphoedema.
Conclusion and recommendations
The aim of this SR was to identify and retrieve existing evidence surrounding HF and CT; another objective was to also present the current landscape and its impact within practice relating to the management of chronic oedema and lymphoedema. The review has retrieved a wide variety of sources, such as primary and secondary research and guidelines, which have been analysed to present three themed challenges. Each challenge has fed into the next to present an overall picture of HF and the use of CT, and also its potential link to chronic oedema/lymphoedema. This has revealed the possibility of applying CT in those with HF, within certain classifications and levels of stability. The application to chronic oedema and lymphoedema may be more limited, but this review presents the possibility, nevertheless. The following recommendations attempt to bridge this gap: the creation of a consensus document focusing upon the application of CT in those diagnosed with HF and lymphoedema; the conducting of a systematic review to establish if further evidence exists within HF and chronic oedema/lymphoedema; and further research to identify possible areas in which CT and its application within those diagnosed with HF and chronic oedema/lymphoedema is investigated.
Key points
- Heart failure is not a straightforward contraindication for the use of compression therapy
- Compression therapy can lead to increases in cardiac pre-load, but this affect diminishes within varying periods with this enhancing cardiac output
- Stages 1 and 2 that are stable within the New York Heart Association indicate that compression therapy may be beneficial
- Stages 3 and 4 that are stable within the New York Heart Association indicate the potential benefit but require the involvement of other multidisciplinary team members.
CPD reflective questions
- What is your understanding of heart failure, and how this can be enhanced?
- What are the main issues faced when initiating compression therapy in those with a heart failure diagnosis in your local area?
- How can you build closer connections with multidisciplinary team members to enhance the care delivered to patients with heart failure?